Abstract
Background: Positive minimal residual disease (MRD) is an established prognostic marker for hematologic relapse, negative hematopoietic stem cell transplantation (HSCT) outcome, and mortality in adults with B-cell precursor acute lymphoblastic leukemia (ALL; Gökbuget N, et al. Blood. 2012;120:1868-1876). In the open-label, single-arm phase 2 BLAST study (N=116; ClinicalTrials.gov, NCT01207388), treatment with blinatumomab, a bispecific T-cell engager (BiTE®) antibody construct that redirects cytotoxic T cells to residual CD19+ blast cells, led to complete MRD response in 88 of 113 (78%) patients after cycle 1 (Gökbuget N, et al. Blood. 2018;131:1522-1531). Median overall survival was 36.5 months. Among patients with Philadelphia chromosome-negative B-cell ALL in complete MRD remission, relapse-free survival was 54% at 18months. In this analysis of the BLAST study, we assessed the health-related quality of life (HRQoL) of patients during and after treatment with blinatumomab.
Methods: Eligible patients (≥18 years) had B-cell precursor ALL in first or later hematologic complete remission and persistent or recurrent MRD ≥10-3 after ≥3 blocks of intensive chemotherapy. Blinatumomab 15 μg/m2/day was administered by continuous intravenous (cIV) infusion for 4 weeks, followed by a 2-week infusion-free interval, for up to 4 cycles. Patients could receive HSCT any time after cycle 1. HRQoL was assessed using the EORTC QLQ-C30 Questionnaire at baseline, on day 29 of each treatment cycle, at the safety follow-up visit (30 days after end of treatment), and at the efficacy follow-up visits (3, 6, 9, 12, 18, and 24 months after treatment start). The questionnaire included 1 global health status scale, 5 functioning scales (physical, role, emotional, cognitive, and social functioning), 3 symptom scales (fatigue, nausea and vomiting, and pain), and 6 single-symptom items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). For global health status and functioning scales, a higher score indicates better HRQoL; for symptom scales/items, a lower score indicates better HRQoL. A 10-point change is often viewed as the minimum clinically important difference (MID) in EORTC QLQ-C30 (Zikos E, et al. EORTC, 2016). In this analysis, the mean (SD) and the mean (SD) change from baseline to end of cycle 1 of the scores for each scale/item was summarized at each scheduled assessment during and after blinatumomab treatment.
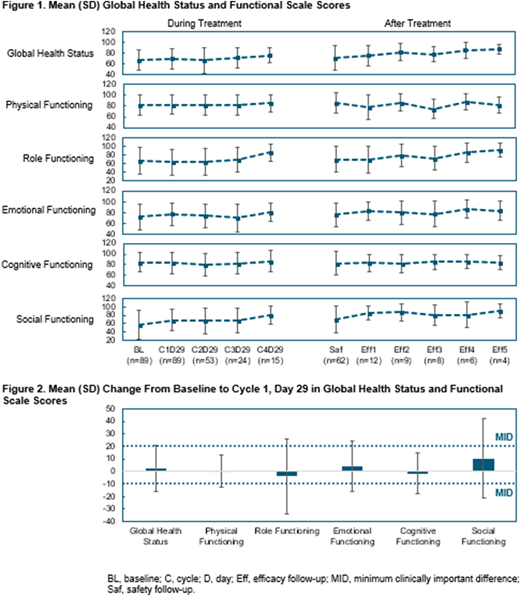
Results: In total, 89 patients had a nonmissing baseline value and a nonmissing value of any scale on day 29 of cycle 1, and thus were evaluable for HRQoL. The patient-reported global health status and functioning scale scores were stable over time during and after blinatumomab treatment (Figure 1). Symptom-scale and single-symptom scores were similarly stable during and after treatment (not shown). Mean (SD) changes from baseline to end of cycle 1 in global health status and in physical functioning, role functioning, emotional functioning, cognitive functioning, and social functioning were 2.5 (18.5), 0.3 (12.5), -4.0 (30.0), 4.2 (20.5), -1.7 (16.2), and 10.4 (31.8), respectively (Figure 2). These results show that, after 1 cycle of blinatumomab, the change in HRQoL was minimal for most scales, with potential clinically meaningful improvements in social functioning. Similar minimal changes were observed for all symptom scales/items (not shown).
Conclusions: In this population of patients with B-cell precursor ALL and MRD successfully treated with blinatumomab 15 μg/m2/day cIV for up to 4 cycles, HRQoL was maintained during and after blinatumomab treatment, which is an important result considering the potential HRQoL impact of standard chemotherapy.
Zugmaier:Amgen Inc.: Consultancy, Employment, Patents & Royalties: 20170327581, 9688760, 20170122947, 9486475, 20160208001, 9192665, 20150071928, 8840888, 20140227272, 20140228316, 20130323247, 20130287774, 20130287778, 20110262440, 20100112603, 7700299, 20070037228. Bonifacio:Incyte: Consultancy; Pfizer: Consultancy; Amgen: Consultancy; Novartis: Research Funding; Bristol Myers Squibb: Consultancy. Topp:Boehringer Ingelheim: Research Funding; Regeneron Pharmaceuticals, Inc.: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding. Tran:Amgen Inc.: Employment. Zhang:Amgen Inc.: Employment, Equity Ownership. Goekbuget:Kite / Gilead: Consultancy; Celgene: Consultancy; Novartis: Consultancy, Other: Travel support, Research Funding; Pfizer: Consultancy, Other: Travel support, Research Funding; Amgen: Consultancy, Other: Travel support, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal